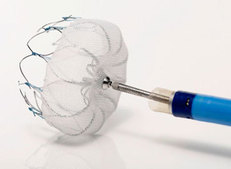
 On March 13th the Food and Drug Administration finally approved the WATCHMAN device for prevention of stroke in patients with atrial fibrillation (AF) in the USA. The WATCHMAN is a percutaneous implantable device designed to occlude the left atrial appendage (LAA). It's been a long journey for this device, now owned by Boston Scientific and six years have passed since the publication of the first clinical trial, PROTECT-AF. In AF 90% of cardiac thrombi are thought to originate in the LAA. A strategy to occlude or remove the LAA is an attractive target to reduce stroke risk and avoid the need for lifelong anticoagulation. Already some cardiac surgeons routinely remove the LAA at the time of cardiac surgery. There is a clinical trial, which will report in 2016, testing the hypothesis that this additional surgical procedure will reduce the risk of stroke. Even if this trial is positive however it will only be relevant to patients undergoing cardiac surgery for other reasons and not open to the vast majority of patients with AF. The PROTECT-AF trial showed the device was non-inferior to warfarin for stroke prevention but there was a high rate of serious procedure related complications. Interestingly when the data was analysed looking at earlier versus later implants the complication rates decreased markedly as operator experience increased. This was confirmed in a later follow-up registry study and further date came from another clinical trial called PREVAIL. If you want to read all of the clinical trial data in detail then the summary on the FDA website is excellent. The clinical trials with the LAA device are very small in comparison to modern pharmaceutical trials. For the NOACs in AF we have four mega-trials and a meta-analysis with 102,000 patients worth of date. Compare this to the PROTECT-AF trial with its mere 707 patients. It is therefore not surprising that the confidence intervals in this device trial are very wide. Interestingly the major benefit in the PROTECT-AF trial was the reduction in the risk of intracranial bleeding. Since this safety endpoint is significantly reduced in patients taking NOACs it would be more appropriate to be considering comparing the LAA occlusion devices with NOACs rather than Warfarin. Now the device has been approved by the FDA we will see a surge in the rate of implants in USA. In Europe already the implant rate is very high in Germany a phenomenon very commonly seen in this country when it comes to the treatment of patients with devices and interventional procedures in cardiology. In the UK some centres such as the Royal Brompton Hospital have implanted over 100 devices. Interestingly NHS England's specialist commissioning have recently selected two different tertiary centre units to perform this procedure in London. Perhaps they didn't consider that the association with operator experience and procedural complication rates was important. Personally I think that the LAA occlusion device will end up as a niche procedure which has a role for those patients with a high CHADSVASC score who are unable to take oral anticoagulants. It is vital going forward that more research takes place with the WATCHMAN and other LAA occlusion devices to determined whether their potential promise will be delivered. At this point we are right to ask Quis custodiet ipsos custodies?
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
Dr Richard BogleThe opinions expressed in this blog are strictly those of the author and should not be construed as the opinion or policy of my employers nor recommendations for your care or anyone else's. Always seek professional guidance instead. Archives
August 2023
Categories
All
|
 RSS Feed
RSS Feed

