 They were a long time coming. We all knew there was a potential for a blockbuster drug one that would make the next billion dollars for its Big Pharma parent. This was going to be “Better than the Beatles”, this was the Stones. There was a false start with Ximelagatran but then with the RELY-AF trial and dabigatran the world changed. Having put up and shut up for 60 years it seemed as though finally warfarin would be consigned to the graveyard of drugs along with old favourites such as guanethidine, reserpine and paraldehyde. The NOACs or new oral anticoagulants were born. They were new, they were oral and they were anticoagulants. However just a minute isn’t there a problem with the NOAC acronym. They won’t always be new will they? They have already been around for 5 years and we are still calling them new. Will they still be new in another 5 years? I propose it is time to stop calling them new and rename then "Non-warfarin oral anticoagulants." We can still keep the catchy NOAC acronym but the new name will reflect what the drugs actually do and that they are different from warfarin. There a subtly in the current acronym which I believe is impeding the widespread adoption of these medicines. The word "new" implies just that. New, therefore we don’t know much about them. New, they are mysterious, perhaps dangerous? The word needs to be dropped. Health economies put up restrictions to impede implementation of new medicines and in the UK the current model of drug development is broken. It was based on the blockbuster and the ability to make a billion dollars from your medicine. It relied on a rapid uptake of the medicine after launch. We now talk about cost-effectiveness of medicines. This is what NICE tests with its unofficial acceptable cost per QALY of £30,0000. What of affordability though? Each health economy has to decide but if you as the patient need the drug and believe it will help me then you don’t really care about the cost per QALY. You probably don’t care about the local health economy or what can and can’t be afforded–you want the drug. Recent headlines about prostate and breast cancer are cases in point. The Cancer Drugs Fund – designed to fund drugs that NICE does not deem cost effective shows that emotion and opinion rules rather than evidence. After all the £30,000 threshold is not scientifically derived it is a matter of opinion and other countries would deem the figure too low after all what price life? There is a subtle trend for the NHS to try and delay and restrict implementation of new medicines for as long as possible. The 2014 NICE guidelines on statins tells us that atorvastatin 20mg is now the preferred statin for primary prevention and 80mg for secondary prevention. This is not because of new evidence – the data has been around for years. The reason is simply that of economics. When atorvastatin was a branded medicine costing £29 per month it wasn’t deemed to be worth it – simvastatin was cheap and therefore more cost effective and more affordable. Now the argument is swept away because atorvastatin is generic and as cheap as simvastatin. We will see the same argument with the NOACs. When they become generic the arguments about effectiveness will all change. The drugs are simple to use as, if not more, effective and don’t require a trip to the anticoagulation clinic. If they were the same price as warfarin there would be no reason not use them. The patent on dabigatran expires in just three and a half years (February 2018) and that is the earliest we will see a generic version appear. At that point use of warfarin for atrial fibrillation and most other indications is likely to cease as the price of the non-warfarin anticoagulants crashes. Will we then stop calling them new oral anticoagulants?
0 Comments
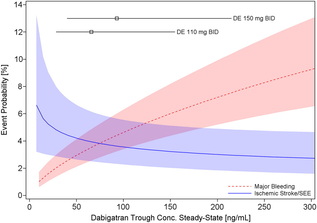 Probability of Major Bleeding Event and Ischemic Stroke/SEE Versus Trough Plasma Concentration of Dabigatran Calculated for 72-year-old male atrial fibrillation patient with prior stroke and diabetes. Lines and boxes at the top of the panel indicate median dabigatran concentrations in the RE-LY trial with 10th and 90th percentiles. Only time will tell as we get a more complete picture of the clinical pharmacology and real world experience of using the NOACs but like all new drugs we should exert a degree of caution and remember that we only know about half of what we need to know when the drug is launched.
|
Dr Richard BogleThe opinions expressed in this blog are strictly those of the author and should not be construed as the opinion or policy of my employers nor recommendations for your care or anyone else's. Always seek professional guidance instead. Archives
August 2023
Categories
All
|
 RSS Feed
RSS Feed

