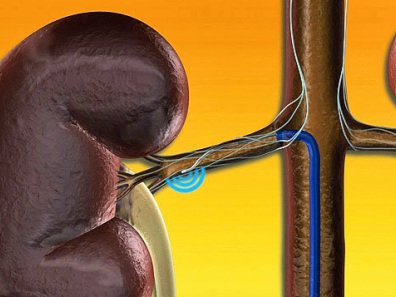
Renal Sympathetic Nerve Ablation (RSNA) for Hypertension
Important Information about Renal Denervation for Hypertension 14th January 2014
Medtronic have announced in a press release that the SYMPLICITY HTN-3 clinical trial did not meet its primary efficacy endpoint. This means the treatment was no more effective than the sham control on reducing clinic measured blood pressure measurement. The trial did meet its safety endpoint therefore indicating no harm to patients from the procedure.
SYMPLICITY HTN-3 was the first blinded, randomized controlled trial designed to evaluate effectiveness of renal denervation in patients with resistant hypertension. The study randomized 535 patients to renal denervation or a sham-procedure with all patients continuing to take their blood pressure medications.
A review published in November 2013 in Heart compared the large reported falls in clinic BP with the smaller falls in ambulatory blood pressure in drug and the renal denervation trial and predicted that the results of the Symplicity HTN-3 trial were likely to be negative. There is also a YouTube presentation by the senior author of the paper which is very informative.
Pending the publication of the results of the Symplicity HTN-3 trial we have suspended the renal denervation programme at St Helier Hospital.
This action is in line with the recent statement published by the Joint UK Societies Working Group on Renal Denervation
Medtronic have announced in a press release that the SYMPLICITY HTN-3 clinical trial did not meet its primary efficacy endpoint. This means the treatment was no more effective than the sham control on reducing clinic measured blood pressure measurement. The trial did meet its safety endpoint therefore indicating no harm to patients from the procedure.
SYMPLICITY HTN-3 was the first blinded, randomized controlled trial designed to evaluate effectiveness of renal denervation in patients with resistant hypertension. The study randomized 535 patients to renal denervation or a sham-procedure with all patients continuing to take their blood pressure medications.
A review published in November 2013 in Heart compared the large reported falls in clinic BP with the smaller falls in ambulatory blood pressure in drug and the renal denervation trial and predicted that the results of the Symplicity HTN-3 trial were likely to be negative. There is also a YouTube presentation by the senior author of the paper which is very informative.
Pending the publication of the results of the Symplicity HTN-3 trial we have suspended the renal denervation programme at St Helier Hospital.
This action is in line with the recent statement published by the Joint UK Societies Working Group on Renal Denervation
A New Therapy for Treatment-Resistant Hypertension
At St Helier Hospital we have developed a multi-disciplinary team to pioneer this recently introduced novel and innovative therapy for treatment-resistant hypertension. Our team consists of Dr Bogle (Interventional Cardiology), Dr Pauline Swift (Nephrologist) and Drs Keane and Burney (Interventional Radiology). The Symplicity renal denervation system is a new, safe, and potentially effective tool to use for patients who, until this point, have been unable to achieve target blood pressure levels despite multiple prescription medications, and are considered treatment-resistant. The renal denervation system has been used since 2007 to treat more than 4,000 patients with treatment-resistant hypertension worldwide. The therapy has been used in clinical trials but results of double blinded and sham controlled studies are awaited.
At St Helier Hospital we have developed a multi-disciplinary team to pioneer this recently introduced novel and innovative therapy for treatment-resistant hypertension. Our team consists of Dr Bogle (Interventional Cardiology), Dr Pauline Swift (Nephrologist) and Drs Keane and Burney (Interventional Radiology). The Symplicity renal denervation system is a new, safe, and potentially effective tool to use for patients who, until this point, have been unable to achieve target blood pressure levels despite multiple prescription medications, and are considered treatment-resistant. The renal denervation system has been used since 2007 to treat more than 4,000 patients with treatment-resistant hypertension worldwide. The therapy has been used in clinical trials but results of double blinded and sham controlled studies are awaited.
| |||||||
|
|
Watch a film about how the procedure is performed. For more information please visit the Medtronic Renal Denervation website. |