 Colchicum autumnale Colchicine from the autumn crocus (Colchicum autumnale) has been used in medicine for 2000 years. It is mentioned in the Ebers papyrus dating from ~1550BC and it was recognised as a treatment for gout in Dioscorides's De Materia Medica in the first century. Colchicine has shown beneficial effects in treatment of familial Mediterranean fever and recurrent pericarditis. More recent studies have investigated whether colchicine decreases atrial fibrillation after ablation and this week a new trial in JACC has shown that colchicine reduces cardiovascular events in patients with chronic stable angina. In stable coornary artery disease a previous study showed that colchicine 0.5mg twice daily reduced CRP levels by 60%; interesting but hardly proof of a beneficial clinical effect. A clinical trial published in JACC this week has taken this one step forward and investigated the effects of colchicine in patients with stable angiographically proven coronary artery disease. The Low Dose Colchicine (LoDoCo) study investigated 532 patients randomized to open label colchicine (0.5mg per day) treatment or no colchicine for two years. After a 3 year median follow-up the primary outcome (acute coronary syndrome, out-of-hospital cardiac arrest, or noncardioembolic ischemic stroke) occurred in 16% of placebo versus 5.3% of colchicine treated patients (67% significant RRR; NNT 11). The primary endpoint was driven by the reduction in ACS events as you might expect. On the safety side there was an 11% drop out rate from the colchicine treated group due to adverse GI effects of the drug during the first 30 days of treatment. The mechanism of action of colchine in reducing ACS events is unclear and the authors postulate it is secondary to an anti-inflammatory effect reducing cytokines and certaining this trial provides some support for the paradigm that reducing inflammation is beneficial in ACS. However we are still a long way from being able to conclude that colchicine is a therapy for stable CHD patients. A larger double blind clinical trial is needed before it could enter clinical practice for this indication. But who would fund such a trial with with a drug that is generically available. This would seem like an ideal trial for an investigator led study funded from MRC/BHF or Wellcome. However it might just interest the Pharma and in this respect colchicine has history. In July 2009 the FDA officially announced that colchicine was effective in treating acute gout. But didn't we know that 1500 years ago! Well colchicine had never been officially approved by the FDA. Although the 1938 Food, Drug, and Cosmetic Act required new drugs be approved it allowed drugs already marketed to remain available. Colchicine was one of a number of drugs that the FDA never formally evaluated. In 2007 URL Pharma did studies with colchicine to investigate the drug's safety and efficacy in gout in a randomized controlled trial. On the basis of their results the FDA approved Colcrys (the URL brand of Colchicine) for treatment of acute gout. Because this was technically a new indication for the drug, the Hatch-Waxman Act authorized the FDA to give the company 3 years market exclusivity which led to the price of colchicine to rise 50-fold from 9 cents to $4.85 per tablet. Ample enough reward considering the trial only had 185 participants with. A appropriately powered trial of colchinine in stable coronary disease would require a considerably greater number of participants and long period of follow-up so the rewards to Pharma might not be enough to make this viable. So colchicine remains an interesting drug and 2 millenia after its discovery we are still learning new things about its pharmacology but whether it will enter the cardiologists arena for treatment of coronary artery disease remains to be determined. Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease. JACC 29 Jan 2013: Vol. 61, pp. 404-410. Incentives for Drug Development — The Curious Case of Colchicine. N Engl J Med 2010; 362:2045-2047
0 Comments
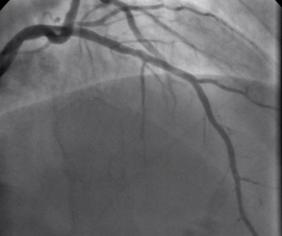 Non flowing limiting mid LAD stenosis Most patients who present with an acute coronary syndrome (ACS) undergo coronary angiography and the majority are then treated with percutaneous coronary intervention (PCI). The remaining patients either have coronary artery disease which is not suitable for revascularisation or have lesions which do not appear to be angiographically flow limiting. ACS is thought to arise following rupture or erosion of thin-cap fibroatheroma (TCFA) on vulnerable plaques. When the angiogram shows non-flow limiting, but irregular and hazy lesions, some cardiologists feel uncomfortable treating the patient with medication alone. There is often discussion in the catheter laboratory about whether a coronary stent should be deployed with the rationale that this might "seal" or stabilise" the plaque and reduce the chance of future cardiac events. Advanced imaging with intravascular ultrasound-virtual histology (IVUS-VH) or optical coherence tomography (OCT) may help to identify TCFAs but there is no evidence that treating such lesions with bare metal or drug eluting stents reduces furture coronary events. Any potential benefit of stent treatment needs to be balanced against the risk of procedural complication, re-stenosis and stent thrombosis. A recent trial has sought to address the question of how to treat the vulnerable plaque. The SECRITT study published in Eurointervention in December 2012 investigated the effects of a stenting vulnerable plaque. 23 patients with high risk IVUS-VH and OCT proven TCFA and a non-flow limiting lesion proven with quantitative coronary angiography and FFR by pressure wire were randomised to treatment with a nitinol self-expanding vShield stent. This device has ultrathin 56 micron struts designed to reduce vessel damage and encourage laminar flow. The stent is self expanding and this avoids the need to deploy using conventional high pressure balloons. Following randomisation patient received either the vShield stent (n=13) or standard medical therapy (n=10). The baseline stenosis in the vShield group was 33.2±13.5% and the FFR 0.93±0.06. At six-month follow-up vShield patients had 18.7±16.9% stenosis and FFR was unchanged. The fibrous cap thickness at baseline was 48±12µm increasing to 201±168µm. No dissections occured and there were no plaque ruptures with the VShield. There were no device-related major adverse cardiovascular events (MACE) events at six-month follow-up. In the control group of 5 patients the % diameter stenosis, FFR were unchange at 6 months and there was no significant difference in late loss between the Vshield and medical treated groups. SECRITT is proof of principle study which has demonstrated that passivation and sealing of TCFA with a vShield self-expanding nitinol device appears feasible and safe. Whether treatment of vulerable plaque with conventional stents would have the same results is unknown. A comparison of the VShield with conventional balloon expandable stents has shown conventional stents result in a high proportion tissue prolapse or intra-stent dissection visible with OCT which are less frequently seen with the VShield stent. However, these vessel-wall injuries were not associated with in-hospital clinical events and currently it is difficult to know if OCT-detectable acute vessel-wall injury after stenting is associated with untoward clinical safety events. A long-term, larger randomised study is needed to evaluate the efficacy of stenting the vulnerable plaque is needed, until we have that data intensive medical therapy remains the standard treatment for non-flow limiting lesion. SECRITT Trial Slide Set Comparison of Acute Vessel Wall Injury After Self-Expanding Stent and Conventional Balloon-Expandable Stent Implantation: A Study with Optical Coherence Tomography |
Dr Richard BogleThe opinions expressed in this blog are strictly those of the author and should not be construed as the opinion or policy of my employers nor recommendations for your care or anyone else's. Always seek professional guidance instead. Archives
August 2023
Categories
All
|
 RSS Feed
RSS Feed

